Redox reactions are the movement of either hydrogen(H+) or an electron (e-) from one compound to another. This movement releases energy, which drives forward a biological reaction. Examples of cycles that utilize redox reactions are glycolysis, oxidation of pyruvate, fatty acid breakdown, Krebs cycle, and oxidative phosphorylation.
The formation of glucose is a prime example of a redox reaction, where water is oxidized, and carbon dioxide is reduced electrons are transferred (H+) from water. In aerobic metabolism, glucose can be oxidized.
Photosynthesis = Energy Creation
Sunlight Energy + 6CO2+6H20 —————— C6H12O6+6O2
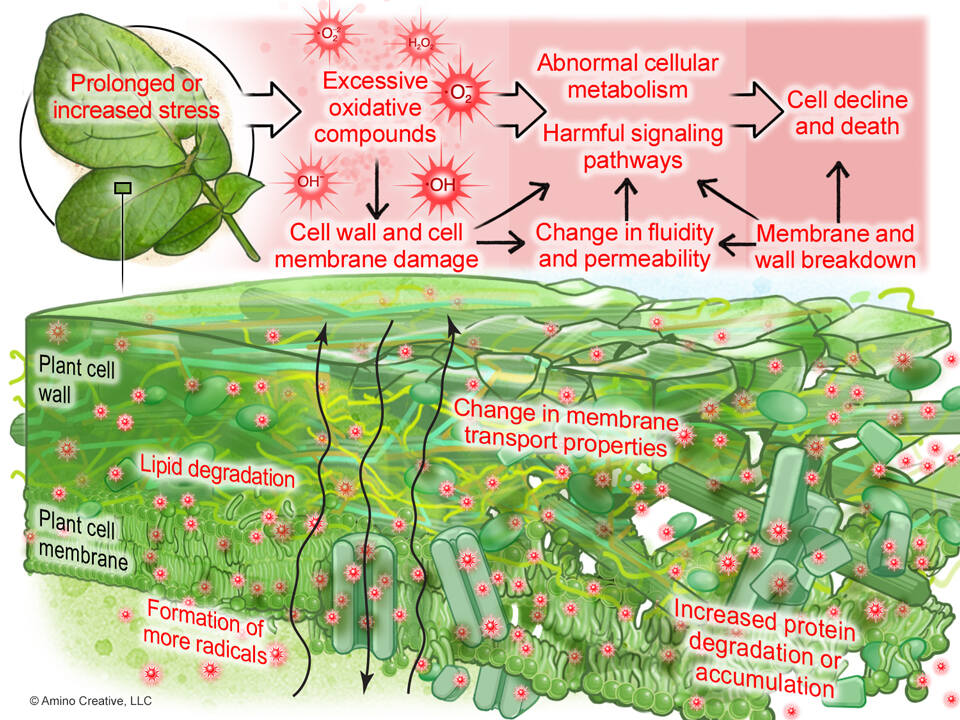
Aerobic metabolism in plants produce oxygen, carbohydrates, and Reactive Oxygen Species (ROS). Positively, ROS act as signaling molecules, driving forward several metabolic pathways in plants (cell proliferation and differentiation, programmed cell death, seed germination, gravitropism, root hair growth and pollen tube development, senescence). Negatively, ROS will cause structural damage to proteins, cell walls, DNA, and other critically important complex biomolecules. This damage occurs during abiotic stress events such as excess light, high heat, excess cold, high salinity, nutrient imbalance, chemical damage, physical damage, or potentially could lead to biotic damage.
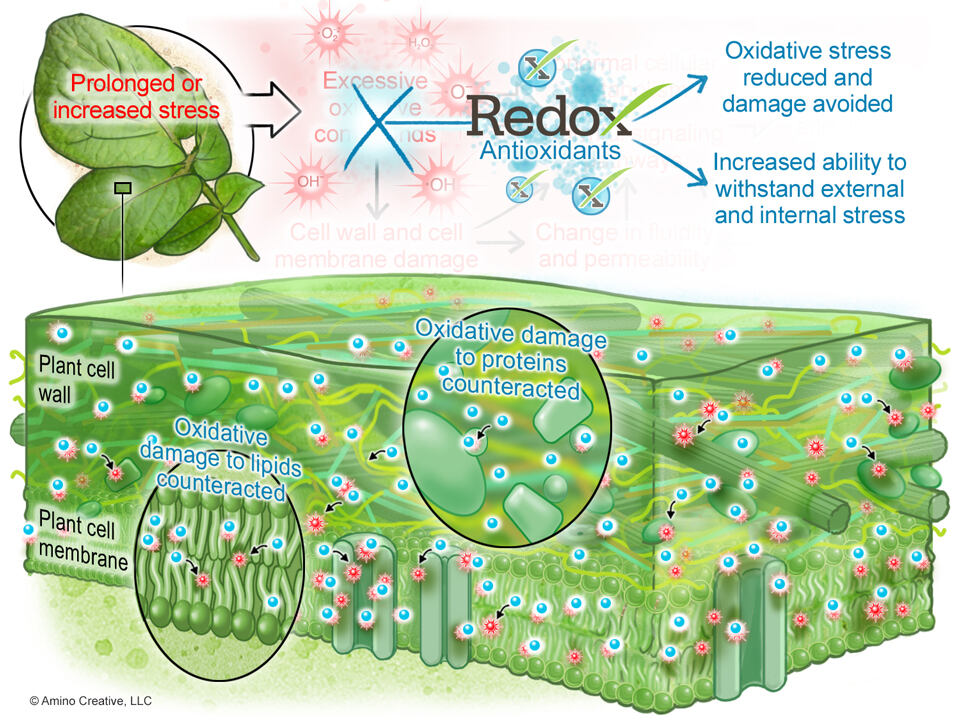
Antioxidants reduce the damage of excessive ROS by acting as either an electron donor or an electron acceptor, thus reducing the potential damage of oxidants.
Antioxidants may be produced by the plant, by soil microorganisms, and thru bio-nutrients.
Subscribe to receive our Redox Bio-Nutrients updates